As noted in the nuclear energy theory page, fissile materials are particular isotopes of Uranium and Plutonium.
- Uranium
Uranium, the heaviest naturally occurring element, is 40 times more abundant in the Earth's crust than Silver and is about as common as Tin or Zinc. Naturally occurring Uranium is 99.2745 percent Uranium-238, with Uranium-235 the fissile isotope used in most reactors making up only about 0.720 percent, and Uranium-234 filling in the remainder at less than 0.0055 percent.
The Uranium fuel is normally used in its ceramic Uranium oxide form which has a melting point of 2800°C and for most applications the percentage of the fissile Uranium-235 is enriched to increase the probability of neutron capture thus facilitating the fission process. Using enriched Uranium also allows the reactor core to be made physically smaller than the core needed for an unenriched Uranium reactor.
The target percentage of U-235 used in the typical light water reactors used for electrical power generation, is from 3% to 5% of the total Uranium charge. For weapons grade Uranium however the concentration is much higher at around 85% to 90%.
-
Production
Extraction
The initial processes take place near to where the Uranium is mined. Uranium ores are crushed into small particles about 1 cm diameter and treated in a leaching process with steam, sodium chlorate and sulphuric acid to dissolve the Uranium out of the rock.
The resulting aqueous solution is decanted and filtered and then concentrated, first into an organic phase by treatment with various organic solvents, then further concentrated into a second aqueous phase and finally precipitated into a solid oxide form by treatment with Ammonia. After filtering and drying this solid Uranium oxide (U3O8 ) is known as yellowcake.
ConversionThe rest of the fuel preparation may take place nearer to where the fuel is used.
EnrichmentOnly 14% of all reactors use natural Uranium fuel, whereas 85% use enriched fuel and 1% use other fuels.
The process of "enrichment" to concentrate the percentage of the isotope U-235 in the fuel involves differentiating between the isotopes present in the refined material on the basis of differences in their physical properties. The separation process is thus based on the mass and size of the molecules and since these differences are minute, the processes used involve many repetitive stages to achieve appreciable separation.
Practical enrichment processes need the fuel to be in gaseous form. The yellowcake must therefore be converted, via a series of chemical process steps, into Uranium hexafluoride UF6 which is the only compound of Uranium which exists as a gas at a suitable temperature. At atmospheric pressure UF6 is a is a white, dense, crystalline solid resembling rock salt below a temperature of 57°C and transforms directly from a solid to a gas at that temperature without going through a liquid phase. Liquid UF6 is formed only at temperatures greater than 64°C and at pressures greater than 1.5 times atmospheric pressure.
-
Gas Centrifuge
The UF6 gas is rotated at extremely high speeds of 100,000 rpm or more in a centrifuge and due to the centrifugal force the heavier U-238 isotopes tend to move towards the outside increasing very slightly the concentration of the heavier isotopes at the periphery compared with a slightly higher concentration of the lighter U-235 isotopes nearer the centre. The gases are withdrawn and the heavier gases are passed through a series of centrifuges to concentrate the proportion of U-238 while the lighter gases are recycled back to lower stages to concentrate the proportion of U-235.
Gaseous DiffusionIn the diffusion process the UF6 gas is passed through a series of several hundred sets of very fine membranes. Separation depends on the lighter U-235 isotopes passing more quickly through the barriers than the larger U-238 isotopes.
The holes in the membrane must be microscopic (approximately one-millionth of an inch in diameter) and uniform in size. The porosity must always be high to enable high flow rates and the membrane must not react with the highly corrosive hexafluoride.
After the enriched Uranium has been separated from the natural fuel, the percentage of fissile Uranium-235 remaining in the so called Depleted Uranium is reduced to between 0.025% and 0.03%, the rest being fertile Uranium-238 which can be used in breeder reactors to create more fuel.
Fuel Charge ProductionOnce the UF6 gas has been enriched the Uranium must be converted into a form suitable for use in the nuclear reactor. This is generally as Uranium dioxide UO2 since in this metallic oxide form it is chemically stable up to temperatures over 2000°C, high enough to survive the high working temperatures in the reactor core.
First the gas is converted into a powder of UO2 which is subsequently sintered to form small pellets about 10mm in diameter and 10mm high.
Fuel CanistersFuel canisters must be able to withstand high temperature working and have high mechanical strength with low neutron absorption characteristics
In large Light Water Reactors (LWR) and Pressurised Water Reactors (PWR), pellets of enriched uranium oxide arranged in rods of zircaloy an alloy of Zirconium. Early Gas Cooled Reactors (GCR) used magnesium alloy to contain the fuel but this was replaced in later reactors by stainless steel which is able to withstand higher temperatures.
Uranium SuppliesThe world's present measured resources of Uranium are enough to last for about 100 years at current and projected consumption rates. This represents a higher level of assured resources than is normal for most minerals. Further exploration and higher prices will certainly yield further resources as present ones are used up.
PlutoniumPlutonium is produced by bombarding Uranium-238 with both slow and fast neutrons.
Also bombarding Uranium with deuterons, the nuclei of the Hydrogen isotope Deuterium containing one proton and one neutron.
-
Production
Huge diffusion plants like those used to enrich Uranium-235 are not needed for the production of Plutonium since it is produced in large quantities in breeder and other reactors and is relatively easy to separate chemically from Uranium.
See also Breeder Reactors below.
A major safety system in nuclear reactors is provided by control rods of Boron, Cadmium or Graphite which absorb neutrons created by the fission process removing them from the active mass thus preventing further fissions from taking place. Because of their atomic structure these elements absorb neutrons, but do not fission or split. The rate of the chain reaction can be controlled by progressively inserting the control rods into, or removing them from the reactor core and the reactor can be quickly shut down by dropping the control rods into the core.
ModeratorsThe energy of the free neutrons must be within certain limits for for fission to occur. High energy neutrons emitted by the fission process move too quickly to be captured by the fissile atoms and so must be slowed down or moderated to increase their chances of causing fission. Water, heavy water and graphite are moderators which are commonly used in the reactor core to slow down the neutrons. Certain hydrides, hydrocarbons, beryllium and beryllium oxide are also used for this purpose.
Note that some moderators can also act as coolants.
-
Thermal Reactors
Reactors with moderators are called thermal reactors.
Fast Neutron ReactorsReactors without moderators are termed Fast Neutron Reactors because the speed of the neutrons is not controlled.
The reactor core acts as a heat exchanger in which the coolant, which may be either a liquid or a gas, surrounds the fuel rods and captures the heat generated by the nuclear reaction. The coolant also acts as the thermal working fluid which is used either directly or indirectly to raise steam to drive a turbine generator.
Coolants must be good conductors of heat with low susceptibility to induced radioactivity and capable of operating at high temperatures. A variety of substances, including light water, heavy water, air, Carbon dioxide, Helium, molten metals such as Sodium, Sodium-Potassium alloy, Lead and Lead-Bismuth alloy as well as hydrocarbons (oils), have been used for this purpose.
-
Containment
Reactors are contained inside a huge reinforced concrete casing often incorporating a steel inner structure which acts as a radiation shield and is designed to prevent the release of radioactivity into the environment in case of an accident in the reactor as well as to protect the reactor from external events such as earthquakes, aircraft impacts and deliberate acts of sabotage.
The notorious meltdown of the Chernobyl nuclear reactor in 1986 was initiated by operator malpractice which inadequate safety systems failed to prevent. Because the reactor was not enclosed in a containment building, vast areas of the countryside were contaminated with deadly radioactive debris.
By contrast, in the 1979 accident at Three Mile Island when the reactor core went into partial meltdown and was destroyed when the cooling system failed due to the loss of coolant, the radioactive debris were successfully contained within the containment building.
The Reactor Thermal CircuitsCooling is another major challenge in reactor design. Heat is extracted from the reactor core by one or more tightly controlled, closed, heat transfer circuits and used to power a conventional steam or gas turbine generator. Many variations are possible.
-
The Rankine Cycle
The Rankine cycle describes a thermodynamic power cycle in which the working fluid is alternately vaporized and condensed as it recirculates in a closed cycle. It is similar to the Carnot cycle the except that it takes into account the energy absorbed and returned by the reversible liquid/gas phase changes which reduce somewhat the efficiency of the thermal cycle.
The Rankine Efficiency is proportional to (1-TL/TH) where TL is the fluid temperature at the low heat point in the cycle the output temperature and TH is the fluid temperature at the high heat point, the input temperature. As with the Carnot cycle, the thermal efficiency is improved by maximising the temperature difference between the input and output points.
The thermal cycle used in steam engines is an example of the Rankine cycle.
The Brayton (Joule) CycleThe Brayton cycle, sometimes called the Joule cycle, describes the thermodynamic power cycle associated with the compression and expansion of a gaseous working fluid. Analogous to the Carnot cycle the thermal efficiency is maximised by increasing the pressure difference between the input and output points. The Brayton cycle is used to represent the thermal cycle used in gas turbines.
In gas cooled nuclear reactors in which the gas coolant is used directly to drive the turbine, heat from the reactor increases the pressure of the gas in the reactor heat exchanger and the pressurised gas gives up its energy by expansion in the turbine.
Like the Carnot cycle the Brayton cycle does not encompass a phase change and hence it has the potential for higher efficiencies.
Single Stage Heat TransferIn single stage cooling systems the reactor coolant or thermal working fluid, either steam or in some cases gas, is used directly to drive a turbine generator.
The boiling water reactor (BWR) is typical of a single stage system. It uses a single water circuit in which the steam is generated directly in the reactor core and used to drive the turbine.
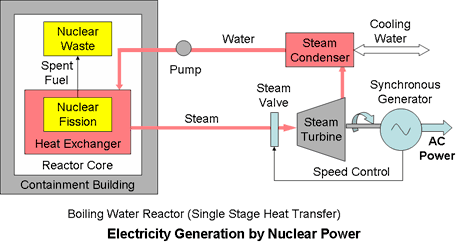
It is a relatively simple design in principle, characterised by the Rankine thermodynamic cycle, but it needs complex control systems to ensure safety. This has the disadvantages that mildly radioactive coolant from the reactor core passes outside of the containment building and that radioactivity can build up in the turbine. The Fukushima nuclear power plants in Japan damaged by the 2011 earthquake and tsunami were boiling water reactors.
Despite the high technology steam generation, the system efficiency is still bound by Carnot's Law and limited by the maximum temperature difference achievable in the steam cycle.
Typical system efficiency is 33% to 36%.
Two Stage Heat TransferFor safety reasons a two stage system is employed to separate the thermal circuit used to drive the steam turbine from the primary thermal circuit which removes the heat from the reactor. The heat generated by the reactor is not used directly to raise steam to drive the turbine generator. Instead, the working fluid in the primary (reactor) circuit transfers its heat through a second heat exchanger to a secondary circuit which is essentially the same as the steam turbine thermal circuit used in a conventional fossil fuelled electricity generating plant but with the steam raising boiler replaced by the secondary heat exchanger. In this way the possibilities of escape of radioactive materials due to leaks of the coolant which has passed through the reactor core can be limited to within the containment building.
This configuration also allows more flexibility in the choice of the reactor coolant so that the working fluid in the primary (reactor) heat transfer circuit may be water, gas or a molten metal.
The added complexity of the double loop cooling system however introduces efficiency losses and extra cost into the system.
The pressurised water reactor (PWR) is an example of a two stage system.
Water at a very high pressure is used as the coolant in the primary circuit and steam is raised in a heat exchanger in the secondary circuit. The working fluid in the secondary circuit is not subject to radioactive contamination.
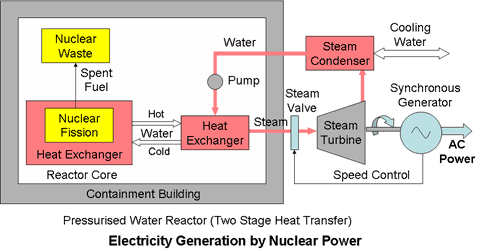
High temperature reactors using molten metal coolants in the primary circuit may use Helium in a Brayton cycle in the secondary circuit operating at 1000°C to achieve very efficiencies of up to 60%.
Tertiary Cooling Circuits A third thermal circuit is used in both the single and two stage systems to cool the working fluid at the end of the work cycle. This is typically an open cycle employing a conventional cooling tower as used in fossil fuelled power plants.
The system efficiency is similar to the boiling water reactor at 33% to 36%
-
Pressurised Water Reactor (PWR)
Over 60% of all installed commercial reactors are pressurised water reactors and like 85% of all reactors they use enriched Uranium as the fuel. The use of enriched fuel means that a higher power density is achievable in the core and thus better efficiency.
PWR reactors use a two stage heat transfer system with ordinary (light) water acting as both a moderator and the coolant in the primary circuit. The water in the primary circuit reaches a temperature of about 325°C and must be at very high pressures of 1000-2200 psi (70 -150 bar or 7-15 MPa) so that it can not boil. It gives up its heat in a second heat exchanger which produces the steam in the turbine circuit based on the Rankine cycle.
Typical output power is 1000MW with a system efficiency of 33%.
Boiling Water Reactor (BWR)
Boiling water reactors have many similarities to the more complex pressurised water reactor and are used in over 20% of nuclear power installations. They use enriched Uranium fuel and like the PWR they use ordinary light water which acts both as the moderator and a coolant but in a single stage heat transfer circuit. The coolant is maintained at a lower pressure of about 1000 psi (75 bar or 7.5 MPa) so that it boils in the core at about 285°C and the resulting steam is used to drive a steam turbine.
Because of the low steam pressure and temperature the Carnot efficiency of the system is also low at. around 32%.
Typical output powers are up to 1400 MW.
Natural Uranium ReactorsEnriched Uranium was not generally available in the early days of nuclear power development and reactors had to be designed to use natural Uranium as the fuel. Because of the low concentration of mobile neutrons in the unenriched fuel, this placed limitations on the types of coolants and moderators which could be used. The purpose of the moderator is to slow down fast neutrons to enable them to be captured by the fissile fuel, however many materials used as moderators also absorb neutrons thus reducing the probability of fission. For this reason ordinary (light) water is not suitable as a coolant or moderator in reactors using natural Uranium fuel since it absorbs too many neutrons leaving insufficient to allow the initiation of a sustained chain reaction. Coolants which do not absorb appreciable quantities of neutrons are heavy water because the Hydrogen atom has already absorbed an extra neutron to form the Deuterium nucleus and has no real affinity for absorbing another one. Some inert gases with a low neutron affinity and a low molecular density such as Carbon dioxide, Nitrogen and Helium are also used as coolants.
-
Gas Cooled Reactor (GCR)
Gas cooled reactors use a double loop cooling system with the gas coolant in the primary circuit and steam in the secondary, turbine circuit.
Gases which are suitable or use in the primary cooling circuit unfortunately do not provide the capability for slowing down the free neutrons in the core and a separate material must be used to moderate the speed of the neutrons. Graphite is typically used as the neutron moderator in gas cooled reactors but Beryllium is also used. Early designs used an alloy of Magnesium, called Magnox to contain the Uranium fuel and reactors were called Magnox reactors.
Advanced Gas Cooled Reactor (AGCR)Gas cooled reactors have the added advantage that the gas coolant can be heated to higher temperatures than water reaching as high as 650°C enabling higher plant efficiencies of up to 40% to be achieved. Higher temperature operation is made possible by cladding the Uranium-235 in stainless steel tubes but stainless steel tends to absorb neutrons slowing down the chain reactions so the fuel is slightly enriched to 2.5% or 3.5% to compensate.
Conversion efficiencies of over 40% are possible.
High Temperature Gas Cooled Reactor (HTGR)Higher Carnot efficiencies have been achieved using Helium as the coolant to allow increased the working temperatures and pressures. This in turn needed the enrichment of the Uranium oxide fuel to 8% Uranium-235.
The high temperature reactor uses a double loop thermal circuit like the PWR reactor. Single circuit designs, based on the Brayton cycle, in which Helium drives the turbine directly are also possible. The Helium must be maintained at high pressure (1000-2000 psi, 7-14 MPa) to achieve sufficient density for efficient heat transfer.
Canadian Deuterium Uranium (CANDU) ReactorAlso called the Pressurised Heavy Water Reactor (PHWR)
As noted above, heavy water absorbs fewer neutrons and so can sustain the chain reaction with unenriched fuel. CANDU reactors use unenriched natural Uranium oxide fuel in a two stage system similar to the PWR. The the primary cooling circuit uses heavy water under high pressure as both the the coolant and the moderator with temperatures reaching 290°C. As in a PWR, the water in the primary circuit must be maintained under pressure so that it can not boil.
Efficiencies of 33% are typical but systems using very high coolant pressures can take this to 45% or more.
Unlike Uranium-235, Plutonium-239 is fissionable with both slow and fast neutrons. Nuclear reactors designed to use fast neutrons, using Plutonium as the fuel, therefore do not need a moderator. There are however extra demands on the coolants used in fast neutron reactors because they should provide efficient heat transfer and should not slow down the fast neutrons. This requirement can be satisfied by molten metals such as Sodium and Sodium-Potassium mixtures which are used for this purpose. Being transparent to neutrons, fewer neutrons are lost in the coolant which as a consequence does not become so radioactive. Molten Lead is also being used in some reactors since it has the added advantages that it provides excellent radiation shielding, and allows for operation at very high temperatures. It is also inert and thus safer to handle than the chemically reactive Sodium.
Fast neutron reactors can be designed as Breeders which produce more fissile fuel than they consume or simply to as Burners which consume the fissile fuel.
-
Breeder Reactors
- U 238 absorbs fast neutrons to become U 239
- U 239 sometimes beta decays twice to form Pu 239 , which fissions
- U 239 sometimes absorbs another neutron to become U 240
- U 240 beta decays twice to form Pu 240 , which fissions
- U 238 is most abundant isotope, ~99% of all uranium
- Fuel needs much less processing
- Virtually indefinite supply available
- Fuel can be "mined" from oceans
- Pu 239 can be used in nuclear bomb
- Pu is highly toxic and radioactive
- Creates nuclear waste as does U 235 fission reactor
Breeder reactors are designed to produce nuclear fuel in bulk from more abundant non-fissile isotopes thus maximising the production of fuel. They can use slow moving neutrons from thermal reactors using Uranium-235 as the fuel to provide the required neutron irradiation, but they more commonly use fast neutrons from the fission of Plutonium-239, in so called Fast Breeder Reactors (FBR), as the neutron source.
-
The Reaction
Plutonium-239 is produced by neutron irradiation of non-fissile Uranium-238 as an unavoidable side effect in all Uranium fuelled reactors. (Uranium-238 forms the greater percentage of the Uranium fuel charge. See above) This reaction, described in the theory section, is a primary objective of the breeder reactor, (the other is power generation) and for its contribution to be maximised it needs to be maintained by fast moving, high energy neutrons which are more efficient than thermal neutrons in transmuting the fertile Uranium-238 into Plutonium. Since fast neutrons have less probability of capture than thermal neutrons, the more fissile Plutonium-239 is used in preference to Uranium-235 as the fuel to enable the release of enough neutrons to sustain a chain reaction. Furthermore since fast neutrons cause less fission than thermal neutrons, the production of fuel can be enhanced at the expense of the generation of power.
Breeder reactors can also be based on slow moving neutrons released in thermal reactors but the Uranium-235 fuel must be enriched to about 20% or more to maintain the reaction.
The ReactorPlutonium breeder reactors use a blanket of fertile Uranium-238 (depleted Uranium) or Thorium-232 around the core of fissile Plutonium-239. Fission of the Plutonium-239 releases more neutrons into the core than conventional thermal reactors and since the reactor does not use a moderator, these are fast, high energy neutrons. The higher concentration of neutrons in the core is sufficient to maintain the chain reaction while at the same time transmuting the non-fissile Uranium-238 or Thorium-232 in the fertile blanket into Plutonium-239.
In this way the breeder reactor can generate 20% to 40% more fissionable fuel than it consumes.
Fuelling a fast breeder reactor with Plutonium requires a reprocessing plant which can handle large amounts of spent fuel with high Plutonium concentrations. Very few of these reactors have been built due to their expense and the fire hazards associated with sodium coolant.
In the breeding of Plutonium fuel in breeder reactors, an important concept is the breeding ratio, the amount of fissile Plutonium-239 produced compared to the amount of fissionable fuel (such as U-235) used to produce it. In the liquid-metal, fast-breeder reactor (LMFBR), the target breeding ratio is 1.4 but the results achieved have been about 1.2 . This is based on 2.4 neutrons produced per U-235 fission, with one neutron used to sustain the reaction.
The time required for a breeder reactor to produce enough material to fuel a second reactor is called its doubling time, and present design plans target about ten years as a doubling time. A reactor could use the heat of the reaction to produce energy for 10 years, and at the end of that time have enough fuel to fuel another reactor for 10 years.
Breeder Reactors in Summary
Fuel is U 238
Fission process is the same as the U 235 reactor
Breeder process

 Barrie graduated from Birmingham University with a degree in Electrical and Electronic Engineering in 1964. Since then he as has worked at Director level in many branches of the electronics industry including military electronics, telecommunications, computers, automotive and consumer electronics. During the last 10 years he has been involved in the battery business, originally as Chairman of MPower Batteries, a custom battery pack making company in Scotland which he helped to found and later in China where he set up a similar business. He is currently Chairman of CHE EVC, another battery startup company pioneering some interesting new technologies. In his spare time he writes and maintains the Electropaedia web site, a comprehensive knowledge base about batteries and energy sources.
Barrie graduated from Birmingham University with a degree in Electrical and Electronic Engineering in 1964. Since then he as has worked at Director level in many branches of the electronics industry including military electronics, telecommunications, computers, automotive and consumer electronics. During the last 10 years he has been involved in the battery business, originally as Chairman of MPower Batteries, a custom battery pack making company in Scotland which he helped to found and later in China where he set up a similar business. He is currently Chairman of CHE EVC, another battery startup company pioneering some interesting new technologies. In his spare time he writes and maintains the Electropaedia web site, a comprehensive knowledge base about batteries and energy sources.